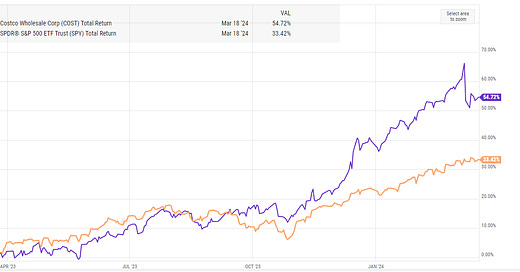
Lionel Hutz discusses his thesis for Liquidia (LQDA) and why the thinks they are likely to win their patent battle against United Therapeutics (UTHR).
This episode is sponsored by Visible Alpha.
While traditional consensus providers offer limited line items and forecast periods, Visible Alpha fills the gap by creating the deepest consensus data in the market, with more and higher quality sources and longer-term forecast horizons.
Through relationships with over 165 brokers, Visible Alpha sets a new industry standard for consensus data across all of a company’s and industry’s products, segments, geographies, and more. Rather than digging through financial models one by one, Visible Alpha extracts data from every line item across sell-side models so professional investors can save time and better understand analyst expectations on metrics beyond just revenue and earnings.
Try Visible Alpha for free by visiting visiblealpha.com/value
Please follow the podcast on Spotify, iTunes, or most other podcast players, as well as on YouTube if you prefer video! And please be sure to rate / review the podcast if you enjoy it, or share it with someone else who would enjoy it (more listeners is a critical part of the flywheel that keeps this Substack and podcast going!).
Disclaimer: Nothing on this podcast or on this blog is investing or financial advice; please see our full disclaimer here. The transcript below is from a third party transcription service; it’s entirely possible there are some errors in the transcript.
Transcript begins below
Andrew Walker: Hello and welcome to the Yet Another Value Podcast. I'm your host, Andrew Walker. If you like this podcast, it would mean a lot if you could rate, subscribe, review it wherever you're watching or listening to it with me today. I'm happy to have on, for the second time, my friend Lionel Hutz. Lionel is a friend, he's a former patent lawyer. You guys might remember him from the Twitter podcast and most importantly, he was the first person to get one of these brand-new Yet Another Value Podcast hat. So, Lionel. How's it going, man?
Lionel Hutz: It's going great. Thank you so much for having me on for a second time. I'm excited to be here and I'm excited to be rocking my Yet Another Value Podcast hat even if your viewers may not be able to see it behind the blurred wall.
Andrew: We're going to blur it out but I appreciate it. It looks beautiful. But let me start this podcast the way I do every podcast. First, a disclaimer to remind everyone that nothing on this podcast invested in us. That's always true but that's going to be particularly true today. We're going to be talking about... Look this is a sub 500,000,000 market cap company so there's extra risk with Liquidia, small size. And then this is, basically, a pre-revenue, micro [inaudible] drug company that's undergoing a patent issue. So, there's just all sorts of added risks here. So, everyone should just remember, please do your homework. There's a lot of risk here. This is an investing advice, consult a financial advisor.
Second, a pitch for you. My guess, people might remember you coming on and we were just pounding the table, "Twitter is going to close to it. Twitter is going to close.". But we've talked about a lot of really interesting situations here. A couple of guests of the podcast, I know, have talked to you about a lot of interesting legal situations here. But you just do great work and I think this is going to be great. This probably going to be our last stock-specific podcast of the year. And I think we saved a great one for last. The company we're going to talk about is Liquidia. The ticker is LQDA. There's so much to talk about with this one. I don't know how sometimes I can't fit a podcast an hour. We could probably take this to a 2 hour podcast, we're going to try not to. I've talked a lot. I'll turn it over to you. What's Liquidia? And why are they so interesting?
Lionel: Yes, Liquidia is a super interesting company, as you mentioned. They are developing a dry powder, inhaled Trepsrostinil for pulmonary hypertension related indications, related instances. Pulmonary hypertension is a pretty terrible group of diseases. The gist of it is you get either inflammation or constricting of the arteries in your lungs and your right heart has to pump extra hard to get blood into your lungs. And patients end up, typically, dying of right heart failure. It's got a pretty high mortality rate, particularly, for some forms of pulmonary hypertension.
And the treatment options are really not great. You have oral medications. You have a couple of inhaled medications and you have injectables. And the options outside of the inhaled drugs that are available are really unpleasant. The oral medications have terrible GI side effects, typically. They're also not capable of delivering super high dosage. So, that means that as the disease progresses, you then have to move to a delivery method, which can actually get you that higher dosage and that is, typically, the inhaled version next. So, you go from an oral medication to an inhaled medication. And that inhaler serves as the bridge before patients, typically, get to the last stage, which is the injections, which are delivered via pump, a subcutaneous pump.
There's a lot of issues around sepsis and pain at injection site. And it's really only used because it's the vehicle that can deliver the highest dosages. And once you're on the injected version, it's sort of just a ticking clock until patients die of this disease or of the related heart failure. So it's a pretty nasty disease and there's just not a lot of great options out there. So, Liquidia is developing...
Andrew: Can I just add one thing?
Lionel: Yes.
Andrew: It's a really nasty disease, but I do think this will be important as people think it is also a very rare disease. So, we're talking about a subset of patients that numbers in the low thousands, if I remember correctly? But it's very rare, it's what's known as an orphan disease. The treatment doesn't need orphan drug but I just think that's important to note for people who are thinking "It's not like one in every four people you run into on the street are having this. This is an awful, awful disease but fortunately, quite rare.".
Lionel: Yes, yes. That's an important note. So, Liquidia is developing, this dry powder, inhaled Treprostinil. The incumbent in the space, which we'll talk about in just a minute, I'm sure United Therapeutics has has approval for, essentially, the same compound, more or less. And it's approved for 2 indications. So, pulmonary arterial hypertension and pulmonary hypertension with interstitial lung disease.
And so, on your point of what are the patient pools over the population sizes, we're looking at something like 12,000 for pulmonary arterial hypertension and something like 30,000 for pulmonary hypertension with interstitial lung disease. Now, I've talked to a couple of pulmonologists who say that those numbers might be a little higher, they might even be double. But again, we're not talking about something that afflicts 2,000,000-10,000,000 Americans [inaudible].
Andrew: Perfect. Yes. That's great. So, Liquidia is developing a drug, UTHR has leader in the space called Tyvaso. I think it's worth noting Tyvaso as of Q3, it is a $1,000,000,000, run rate, orphan drug. They're pricing tens of thousands of dollars for dosage, if I remember correctly. It is a high-cost drug, extremely profitable, brings in a lot of revenue for UTHR. So, Liquidia is coming out with competitor. Right there, you can see there's going to be some drama. You're talking about literally billions of dollars in value. So, where do you want to go next on this? We can talk Liquidia's drug, we can talk about the reason we're here, the patent stuff and everything. What do you think we should hit next?
Lionel: Yes, let's kind of dive into the basics of, I guess, how we got to this litigious stage.
Andrew: Go ahead.
Lionel: So, United Therapeutics has a patent portfolio around Treprostinil that relates not just to the manufacturer of Treprostinil itself, but also the delivery method, the inhalable delivery method. And so, Liquidia, as soon as it was announced that they filed their NDA, their new drug application. And United Therapeutics sees this, "This is a blockbuster drug for us. We want to make sure that we protect our monopoly here.". And so, they sue Liquidia for patent infringement. They sue them just a few months after the NDA was filed and they sue them for 2 manufacturing-related Treprostinil patents.
A month after they file suit, United actually gets another patent issued by the Patent and Trademark Office. And that patent relates to the delivery method. And so, United then goes back and they amend their complaint against Liquidia and they say, "Actually, you infringed not just 2, but 3 patents. You infringe these 2 manufacturing patents, and this 1 delivery-related.". And that then led to all sorts of litigation machinations where Liquidia then says, "Okay, you just sued us in federal district court for patent infringement on these 3 patents.".
But there's a vehicle to challenge these patents outside of this district court process. And that process is called an inter partes review. And that happens at the Patent and Trademark Office. It's where anyone, not just somebody who is an alleged infringer, anyone can go to the Patent and Trademark Office and say, "Hey, you guys shouldn't have actually issued these patents or this patent. It's not valid because of 1 of 2 potential grounds. Either, 1 out of 2 grounds or 1 out of 3 grounds. 1 out of 2 is novelty, 1 out of 3 is not obvious.". So they're saying, "Hey, you, Patent and Trademark Office, you shouldn't have issued this because somebody either did it before or this patent was really obvious in light of these other things that people had done before.".
So, we ended up in a situation where we had, essentially, parallel litigation. You've got the federal district court litigation that United has initiated. And then you've got Liquidia going to the USPTO, the PTAB, the Patent Trial and Appeal Board saying, "Hey, can you guys invalidate these patents?". Now, Liquidia is marginally successful or I should say, they're pretty successful on that front. The Patent Trial and Appeal Board initiates an IPR on one of the manufacturing-related patents. And they decline to initiate an IPR on those manufacturing related patents for reasons that we can talk about in a minute, if you'd like to get into it.
End up being dropped from the district court lawsuit, for a variety of reasons. One of which is that, one of those patents, essentially, gets mostly invalidated at the PTAB. And the other one gets an unfavorable claim construction at a Markman hearing in the federal district court litigation. So, those 2 manufacturing-related patents, end up dropping out of the federal district court suit. And what we're left with is the last patent to be included in that complaint. The delivery-related patent is still an ongoing issue in federal district court and then we also have an IPR initiated on that same patent at the PTAB, which ultimately resulted in an invalidation. So, I'm happy to dive. I know that was a lot, I'm having to dive into any of those elements. It's certainly, procedurally, a lot to kind of digest.
Andrew: It was a lot. So, let me just go through some basics here to confirm, especially, for listeners. This is a generalist podcast so listeners might not have as much patent review. But a lot of people are going to hear patent and they're going to think "Oh, , the company, Merck has patented Viagra, the actual molecule.". This is pretty simple, do they own the molecule or not? That's not what's in dispute here.
What United has is, they've got other things around this drug, the method of delivery, the manufacturing, everything. So, they're not arguing, "Oh, we have the exclusive rights to this molecule.", they're arguing "Liquidia, you're using the molecule in a way that we have patented or you're manufacturing the molecule in a way that we have patented. So, because of that, you can't produce. We're suing you in court saying you can't go and do this until our patents expire.". Is that kind of the right way to talk about it?
Lionel: Yes, it is. And so, a couple of things, if you look at the filings, you'll see for this patent that still is at issue, is the 793, that was the last few digits of the patent. For the 793 patent, it relates to the delivery. So, you see that United is not actually alleging direct infringement. They are alleging induced infringement. The reason for that induced infringement term is because the infringing activity is you using it as an inhaler. And so, Liquidia cannot be a direct infringer because it's actually the patient that is ultimately doing the infringing activity, which is why we see this induced infringement language in the litigation.
Andrew: Yes, perfect. And obviously, you UTHR can't go to every patient and see them and say "You're using the inhaler.", obviously that makes sense. So, 793 patent and we can come back and talk to manufacturing patents later, if we want to. But at this point, the 793 patent, you can yes or no this, tell me if I'm wrong. 793 patent is the one issue at dispute here, really, that's keeping Liquidia from coming on the market or not being on the market, is that about right?
Lionel: Yes, that's correct. So, another kind of stage setting element to all of this is, as soon as the lawsuit was filed, it triggered a 30-month regulatory stay. So essentially, the FDA says, "Hey, Liquidia, you guys can't come onto the market. You can't commercialize until all of this stuff gets resolved.", and so, that stay is still ongoing. Liquidia is not able to commercialize this drug, until we get a resolution in this world of litigation. And again, the 793
patent, the delivery-related patent is the one real outstanding issue.
Andrew: So, what happens here is the 793, at this point, we have 2, kind of, parallel tracks. I guess, they're not working. It's happening at the same time even though they're not technically related. On the one hand, UTHR is suing Liquidia saying, "You violate the 793 patent.", and on the other hand, Liquidia has gone to the Patent Board and said, "We don't think the 793 patent should exist.". And I'll just provide what happens, and then you can provide the story. Because, I think what happens in the story, it's kind of a unicorn case.
Like as you said, when you and I talked offline about this for the first time, you're like, "I haven't seen judge language like this in every...", I'll let you explain that. But I think a lot of sell-side, a lot of [inaudible] have really gotten what happens here wrong because it's a super unique case. So, what happens is, I think in early September, the judge comes out and says, "I am up, basically, and you can give all the details here, I'm upholding the 793 patent. I am ruling that UTHR wins on this. Basically, Liquidia can't come, they were violated.". On the other side, the PTA Board, basically, around the same time, I think it was in July, says, "We are invalidating the 793 patent." for reasons that I will let you discuss.
So, the conflict is, the judge has said the patent is upheld, PTAB has said, "We are in validating this patent.". There's going to be a lot of appeals, there's a lot of process here and we'll go into the timeline and everything. But I just want to set that stage and turn it over to you and say, how should people be thinking about these 2 conflicting rulings? Because I think that's really where the devil and, hopefully, the alpha exists.
Lionel: Yes. We could talk about admin law and what it means to, what, what kind of agency authority the USPTO has for hours. And there are classes taught on this kind of tension that happens, not just with the PTAB, but with other agencies. It's really important to note that the market, from what I've seen certainly, there's analyst report out there that I think was issued last week, that just has it flat wrong, where they said "Oh, the district court litigation overrides the PTAB.", that is an incorrect statement. You essentially have a level of parity between the district court litigation ruling and the USPTO ruling until the Federal Circuit makes their decision on appeal.
So, what is likely to happen is that the PTAB ruling is going to hit the Federal Circuit. Federal Circuit will make their ruling for reasons that again we can talk about in just a second. I expect they're going to uphold the invalidation of the 793. In other words, Liquidia is going to win in my mind. And once the Federal Circuit says, "Yes, PTAB. You got it right. Good to go.". Then that ruling becomes binding on the district court. So, we have this situation right now, where the district court is saying, "Hey, Liquidia, you didn't prove that it is not invalid and we find that you're probably going to induce infringement when patients take this medication.", and the PTAB has said, "Actually, we think it is invalid. And if it is invalid, then Liquidia can't infringe. Because you can't infringe and invalid patent.".
Andrew: Perfect. So, that was great and we can talk about all the complexities behind it. But just to clarify, a lot of people read the judge's ruling and maybe it's because the judge's ruling comes out. Second, maybe it's because you and I did the Twitter case and we know the power of judges here, all this sort of stuff. But a lot of people read the judge's ruling in August that says "Hey, this patent is valid. Liquidia would induce infringement if they launched, right?". They read that and they say, "Oh, UTHR wins.". As you said, there's the sell-side report that says the judge's ruling oversees the Patent Board. And what you are saying is, "No, that is wrong.". If you think about it, logically, if PTAB invalidates the patent, there is no infringement here, right? Because there's no patent to infringe. Am I kind of summarizing that correctly?
Lionel: Yes. I think what people might be latching onto is the fact that because the Federal Circuit has not weighed in yet, it still looks like a United
win here. It looks like UTHR won at the district court and therefore they've won because Liquidia is still not commercializing. The reason Liquidia is not commercializing is because that stay is going to last until the Federal Circuit actually provides an opinion, weighs in on all of this. But it's still inaccurate to say that the Federal Circuit opinion overrides. That is an incorrect statement. And again, something that I think the market has vastly wrong.
Andrew: So, just a very simplified version of this is, at this point PTAB has ruled the 793 patent invalid. Obviously, there's appeals, processes, there's their steps to play out and we'll talk about timeline and everything. But basically, in your eyes, once the 793 patent, once all the appeals play out and, hopefully, it is upheld in Liquidia's favor, that this is not a valid patent. Liquidia will be able to come onto the market.
Lionel: Yes, if all of that rings true, then yes. Liquidia will be commercializing shortly, thereafter.
Andrew: And I want to talk about why the 793 patent's invalid? What is this 793 patent we keep alluding to us? But before we do that, when we first started talking about this a while ago, you said to me that really got me personally, super interested, there were other things, but this is what got me. And that is the judge's ruling on the 793 patent, why it's valid and everything. He's got this really interesting language in it, right? Where he says, "Hey, I'm ruling August 31st on July 19th.", so a month and a half before. "I am aware that the PTAB invalidated this patent. Despite that, I am upholding, I'm saying that Liquidia would violate this patent. I can't do anything about that.", and I read that as a non-lawyer. I'm probably butchering the language. I read that as a non-lawyer. I was like, "Oh, okay. That makes sense.".
And again, this thing that got me super interested, you said, "Oh, no. I could write a whole semester long case in law school talking about what's going on, all the things the judge is saying. That should any lawyer, their hair would just instantly stand up on fire reading this. The judge is signaling and really doing something here.". So I'm going to again, stop rambling and turn it over to you to talk about what the judge was kind of signaling and saying in this August 31st decision.
Lionel: Yes. Well, first of all I don't know if anything I could say as a lawyer is ever going to instigate hair on fire. I think that's...
Andrew: And this isn't legal advice. I mean, we're not giving legal advice out here. We're just kind of [inaudible] [crosstalk] talking.
Lionel: The law can only be so dramatic, right? I think we saw the extreme version of that with Twitter. So, yes. So, Judge Andrews at the Delaware District Court issues his opinion and he says, more or less, "Hey, PTAB. You're not the boss of me.". And I'm not going to stay the litigation and enforcement of my opinion based on Liquidia's... Liquidia says, "Hey, we've got this ruling as PTAB.", judge enters, "Please stay this. Put a pause on this until we resolve this with the PTAB in the Federal Circuit.". And Judge Andrews, he says "No.". Now, his ruling on that is sort of simple. He says "You did this too late.", that's really his primary reason. He says this, "This came too late.".
Normally, if you file for IPR's at the PTAB, a lot of times people will immediately try to stay the the district court litigation. And judges will typically grant that stay because they've recognized it's expensive for the parties to litigate on 2 fronts. And judges don't really want to weigh in on things that are ultimately going to be inconsequential. So, they know that if a patent is invalidated at the PTAB and held up on appeal, that their whole case becomes moot. And so, they just say, "Hey, I'll stay the litigation.". But Judge Andrews says, "You guys moved for this after trial already happened. So, it's too late. Too bad.".
Now, there's another added layer to this because one of the other factors of whether or not a judge, will stay litigation is whether or not the pending issue that they're viewing, externally, whether that will make their case simpler. And for Judge Andrews, the answer to that question is no, because the PTAB is ruling on evidence that wasn't critical to the district court litigation. And we can talk about this, I mean, this is where it really starts to get interesting because the PTAB invalidated the patent, the 793 patent based on 2 key references.
Now I said earlier, the PTAB Can invalidate on novelty grounds or obviousness grounds saying that, "Hey, somebody did this before or somebody did something really similar and you combined these things. It was really obvious.". What's really interesting here is that the 2 references that were used at the PTAB and were not used in the district court litigation, were actually references that were authored by the inventor of the patent. So, what the PTAB is saying is, "Hey, you, the author of the patent...", this guy Voswinkel, "you publish this, you made this known to the world too soon. You published it and then you applied for a patent too late. Too much time had passed.".
In the US, you get a one year grace period as an adventure. You can disclose something to the public and that tolls a one year period, where then you can go to the USPTO and file for a patent. And the PTAB is saying, "Hey, Voswinkel, you publish this and then you waited more than a year. And it looks like Liquidia has shown that these references are one-to-one matches. You just closed exactly this invention that is the foundation of the 793 patent, more than a year before you filed for the patent. So, you Voswinkel, have invalidated your own patent by way of your own disclosures.". And that didn't make its way into the district court ruling. And so, Judge Andrews is saying, "Okay, well this doesn't simplify my case. A ruling on those references doesn't simplify my case because that was not key evidence in my case. And so, I'm just going to keep trucking and we'll see what the Federal Circuit has to say about the PTAB. But we're just going to keep our cases going.".
Andrew: So, another simple way of looking at this is, the patent has been issued. What what Liquidia is kind of saying is the patent shouldn't have been issued because this knowledge was common knowledge, as you said. The inventor, 18 months, 2 years, before you filed for the patent, he had made this common knowledge, he no longer has the right to patent it. The judge is saying, "I can't do anything about that. That's at the Patent Board level. I can only rule on the case in front of me. On the case in front of me, the patent would stand. But if the PTAB rules the patent is invalid. Well, then you can forget about this because the patent was invalid. I'm ruling on something that wasn't there.", is that kind of the right way to think about it?
Lionel: Yes, yes, that's a good summary.
Andrew: Great. So, at this point, on one side we've got the judge who has ruled, "Hey, 793 valid patent Liquidia, you can't release.". We've got the Patent Board who has ruled, "Oh, actually, this is an invalid patent. It shouldn't be an issue because of these things.". I guess, maybe we should talk a little bit more about Liquidia's case for why, as you said, the author had made this public knowledge, why this shouldn't have been, why this patent should have been issued, why it's invalid. Do you think we should talk more about that at this point?
Lionel: Yes, I think it's worth touching on for a bit. So, so these 2 references that Liquidia has found, they call them Voswinkel. Some people call him Jesc, J-E-S-C, and Voswinkel Jaha, J-A-H-A. They are basically 2 references that are abstract, that were published in journals and were associated with related disclosures made at conferences. So, Liquidia is saying, "Hey, Voswinkel, you went to these conferences. You talked about your invention. You had some written abstract where you described it. That abstract was circulated and not only was it circulated but it was actually filed away in a variety of different libraries.", and that's really the important point which we can get into. They say it was filed in all of these libraries. And so, this was a public disclosure. You published these references for the world to see thereby, precluding you from pursuing patent protection.".
Andrew: And just to simplify, every year in New Orleans, there's the American Heart Association or whatever, gets all the top heart doctors together and they do a big... So, basically Liquidia is saying "Hey, you got this great pulmonologist or disease researcher.", whatever. At 2004, he did this big presentation, talking about how to disperse the drug or deliver this drug, right? So he did this big presentation and then in 2006, he filed a patent. And we are saying because he did this big presentation, the patent is not valid. And then we'll talk about how it's in libraries and everything as well. And basically, what UTHR is rebutting, is there saying "No, this conference either wasn't big enough or wasn't well distributed enough.". I don't think they're arguing he didn't do this. They're arguing it wasn't well distributed enough that it became public knowledge. Am I kind of summarizing the 2 sides right there?
Lionel: Yes. So, Liquidia's primary argument is this was available in written publications that were catalogued in libraries. Their secondary arguments are even if it wasn't in this library, you just closed it in a conference and presumably, you handed out paper copies and did all sorts of other things. Their third and final argument is even if those other 2 fail, we see from these other references, we call them research aides, that they cite to these abstracts, which means that they must have been available because somebody else found them.
Andrew: Yes.
Lionel: Now, we can talk about why the secondary arguments and tertiary arguments are not, maybe, as favorable as the primary argument. I think the third argument is the weakest. I wouldn't say this is like dunking on the Liquidia's counsel, Cooley, because I think they're right to put all of the arguments out there. But I think it is a weak argument. I think we can kind of dismiss that because the research aides were actually from after the critical data that is an issue. So, basically, the research aides don't show that these abstracts, these Voswinkel abstracts, we're truly available before the critical data. And so...
Andrew: Basically, if the critical date is March 15th, the research aides were released March 30th. So, there is the possibility. Even though it's crazy to think a scientific paper could be developed in 2 weeks. There is the possibility that the guy saw it March 20th and in 10 days, turned around a giant paper, is kind of the issue with those.
Lionel: Yes, I think, logically, it makes sense that these research aides show was publicly available. Legally speaking, they could have found this, because like you say, if it was March 30th, it could have been published on March 29th even though the conference happened a year before. It could have been finally published March 29th and the critical date was March 15th and it's too late. And so, no it wasn't published...
Andrew: Published March 29th and they pulled the undergrad, "All right, let's get a big cup of coffee and let's get our paper out before the time flies."
Lionel: Yes. "We talked about this a year ago, but we're just gonna get around to publishing it now. And this this other guy's gonna write on it in 24 hours.". Logically, it doesn't make sense but legally speaking, I think it's a weak argument.
Andrew: Yes. So, that's the third argument so let's dismiss that. The second argument is, "Hey, nobody's disputing that he presented this at a conference. Even if it was inside in the libraries...", and I think it we're going to go to the first argument. I think it's so clear we're siding in the libraries and everything, but even if it wasn't, let's put that aside. The secondary argument is he presented at this conference. That's good enough. What do you think on that argument?
Lionel: Yes, we have seminal case on this matter. In re Klopfenstein is this case where the Federal Circuit was grappling with this issue of what does it mean to be a public disclosure to be published if something is presented at a conference. And what they say is if you just speak about something in a conference, we're not going to call that a publishing issue. But if you speak at a conference and everyone there is an expert in your field. So, they're going to digest this, they're going to know what you're talking about, they're going to go back to whatever their research labs and tell everyone else.
And if you hand out abstracts and you're giving materials away to people, then we're probably going to consider this a published reference. And so, here we don't really have all the evidence. It looks like it was probably disseminated in some form. Certainly, it was on early 2000's so, it's unlikely that people were accessing this online at the conference. Yes, we had the internet but like we're people really using their laptops at conferences or were we still in the practice of disseminating papers. I think it's a close call but I think you could argue that Liquidia should win on that.
Again to your point, I think the real critical point here is that Liquidia, in my mind, is going to win on their primary argument which is "Hey, we actually have the physical paper and we found it in these libraries and these aren't Podunk libraries. This is the British Library, the Library of Congress, Stanford, University of Washington. And all of these libraries had appropriate cataloging measures, which means that if you, an expert in this field, a person of ordinary skill in the art, wanted to go find information about whether or not Treprostinil could be inhaled as a dry powder, you could go to these libraries and you could do some searching. And via very standard searching methods, you would actually find these references.".
So, that's the argument that I think is his most profound, is most going to resonate with the PTAB. And certainly, the PTAB ruled in favor of Liquidia. But in their ruling, they just said United Therapeutics challenges Liquidia and says that this primary argument isn't good enough. But Liquidia has these other 2 arguments. And what they didn't say is the primary argument is sufficient. Liquidia wins on argument number 1. And so, we're in this situation where now, the Precedential Opinion Panel at the PTAB, which is a separate little group, that sort of like is a interstitial point between the PTAB and the Federal Circuit appeal, for some cases.
They've said, "Hey, look, PTAB, you need to go back and reissue this opinion. You need to do a rehearing. You need to figure this out because you didn't adequately rule that this was in fact available. You just said Liquidia makes 3 arguments and that's not a ruling. Anybody can tell that they made 3. You need to say which one is sufficient or insufficient? And so that's where we are now, where we're sort of in limbo. The PTAB has ruled 793 is invalid, but they did it in sort of a sloppy way. And now you've got this appeal, that's like ready to go at the Federal Circuit. But the PTAB now needs to reissue an opinion and sort of clarify what they've done.
Andrew: So, you jumped a little bit ahead of me but I like it. So, let's just go back to the original argument and then we'll go to the Precedential Opinion argument. So, just the 793 argument, I just want to drill down for, or sorry, the primary argument for why 793 isn't right? I just want to drill down so people know. So, Liquidia's argument is "Hey...", and there's a librarian expert report that we'll talk about in a second.
But they've got a librarian to test this, she says, "Hey, look. Stanford Library, these libraries...". I never thought about it but the cataloging is so detailed and so specific. She's like "Look, I can go back to Stanford Library and I can search as of what was in their library on November 15th, 2004, which would have been well before the dates we're talking about. I can search and I can find references to them having this specific paper, this specific presentation that he gave in their library.". And she does a bunch of other stuff and they've got it.
As you said, one of the British national libraries has it, but she says five different really prestigious libraries. I can go show that they had this paper in their library and look, if you're a researcher, especially pre-internet, all you did all day was, probably go to a library and look up cardio... I'm studying this drug, let me look up cardiovascular disease with this drug and she says, "A person of ordinary skill would have known to do this. It was in the biggest libraries and the biggest medical libraries in the country. I can prove this, they were available. That's going to invalidate the patent.", is that kind of the right argument on Liquidia's side?
Lionel: Yes, absolutely. And she says this methodology of cataloging, this MARC methodology, M-A-R-K or M-A-R-C, that's a universal standard. You go to these prestigious libraries and it's not like the library is using some ethereal cataloging method. She's saying this is standard. If you go to any library, you're going to see that they're using this kind of MARC method.
Andrew: And what is UTHR's rebuttal to this? I mean, you've got an expert librarian who's coming out and saying, "Look, I can show you in late 2004, 5 different libraries had copies of this abstract, had copies of this paper. What is UTHR's rebuttal to that?
Lionel: I mean, they essentially just say that a person of ordinary skill wouldn't be able to find it, that they're saying that these cataloging measures were insufficient. And what's really important is that they're expert, the person who's saying, "Hey, you couldn't have found this.", is not really a library scientist. Liquidia's expert is a librarian. The expert is deeply familiar with the cataloging methodologies and how you would go about finding this stuff.
And so, you see, in Liquidia's expert's reply brief, she sort of calls out the United expert and says, "Hey, you were like a math teacher and you did all these other things but you weren't a librarian. And so, your opinion is kind of insignificant here. You're trying to tell me that these cataloging methods would be impossible to find these references. And I'm telling you, I've done this my whole career, and it's totally standard.".
Andrew: I'm looking at the Liquidia's expert's brief, and I'm reading these essence and I just love it. It's so funny. And you can talk, maybe, as a former practicing lawyer. You can talk about how these things get drafted and what you kind of read into it. But it's so funny because the expert says, "Look, Wyman...", the UTHR's expert, she's got a Bachelor's in St. John's in Liberal Arts. She's got 2 years worth of post grads in mathematics at UC Berkeley.". Obviously, this is a skilled person, right? But then she comes out and she says, but she is not a trained librarian and it just cracked me up when I read it. But she says, and that is in quotes, "Not a trained librarian.".
But then she says, "Look, trained librarians know how to find these things. They know how its catalogued.", And it is just such a constant dunking on UTHR's expert. "You're not a librarian. The reason you can't find this is because, to your terms or to the legal terms, you're not a person of ordinary skill in this. Yes, you are a very skilled person but you're not skilled in libraries and going through these databases. Any skilled librarian who's got any familiarity with these would be able to figure it out.". And I will just pause there and say you can disagree with anything I said or you can kind of talk about the lawyer things behind how and what gets said in this thing.
Lionel: No, I think that's that's a great summary and when it comes down to it, United's expert basically says, I'm an academic. And so I'm really familiar with finding stuff. But what Liquidia's expert is saying, "Well, you're like a user of the process. I'm like the architect of the process. I'm the person who is in the plumbing of this process.". And so, being a user, just because you drive a car a lot, does not mean you're a mechanic, right? And so, that's kind of the tension that we're pulling at here.
Andrew: If anybody wants to think back to their days in grammar school, walking into a library, you're a user of a library, you might not have been able to find stuff, but you go to the librarian and you say, "Hey, I'm looking for this book.", and she knows exactly where it is because she knows how to use the search system. In a similar way, if you were a drug researcher, maybe you wouldn't know how to find this on your own but you're used to working with librarians saying, "Hey I'm looking for any new materials in the past 3 months on pulmonary hypertension.", and the librarian would have said, "Oh, yes. Let me show you how to find that.". And again, tell me if I'm wrong. Do you want to talk about also how the lawyer game theory of how this gets in and what's it kind of signaling.
Lionel: Yes. I mean, the reality is expert reports... I think, the dirty little secret of these types of litigation, expert reports are usually drafted by the lawyers. Now, they are reviewed extensively by the experts and experts sign off. The experts need to make sure nothing in that is true because they would be perjuring themselves if they agreed to something that was not true. But these are primarily drafted by the lawyers.
And so, what you really have here is Cooley, which is the law firm that is representing Liquidia. You've Cooley kind of calling out United saying, "Hey, you guys picked the wrong person? This is the best person you could come up with for this? Look at our person, our person's great.". So, it's not necessarily that Liquidia's expert is independently saying, "Hey, I'm better than you guys.". It's sort of like Liquidia is saying, "You guys, United, you didn't pick the right person and we did.".
Andrew: And that's the fun dunking. But there's also a little bit of subtext of "Hey, maybe you couldn't find the right person who would disagree with our argument, right? Any trained, librarian would have known this so you guys couldn't go get a trained librarian say I couldn't find that. You had to find a user who said, oh, I couldn't find that.". I don't know if I'm reading too much into this subtext but that's another possible view they're signaling to the judge.
Lionel: Yes, it's totally valid and I think it is worth some weight. Again, being a user of this system certainly is not totally insignificant. But again, you want the person who's familiar with the plumbing and if you've ever done this type of research and I certainly have, I don't know if you have. But having an experienced librarian help you out is fantastic. It is a tremendous skill to be able to find information that is recorded in hard copies.
Andrew: So, I think we've talked about how the librarian, and people can go read the exhibit, I mean, she just, screen after screen of, "Hey, here's a copy of the circular in the British Library and the Library of Iowa.", or whatever it is. Just copy after copy of paste saying that or access stations from library and saying "Yes, our records say we had this and that.", so that's Liquidia. Let's jump to the next thing that you preempted me on, the Precedent Board, if I'm remembering correctly.
They say they read the ruling and they sent it back to the PTAB and says, "Hey, you never explicitly said which ones are valid, which ones are invalid.". So, can you just talk a little bit more about what's going on there? Because I do know, a lot of people saw Precedent Board sends it back to PTAB, and a lot of Liquidia shareholders kind of shook in their pants saying, "Uh-oh.". What was wrong with this, really? I thought the ruling was kind of up the middle. What am I missing here?
Lionel: Yes, so the Precedential Opinion Panel is sort of an intermediate appellate entity where, if the PTAB makes a ruling, and there's a novel issue at stake, a party can basically ask this POP, P-O-P, Precedential Opinion Panel. They can ask the POP to rule on that and essentially, clarify for the Federal Circuit. Because the Federal Circuit Appellate process is long and intricate and so, there's a new issue, you want sort of a beefed-up record. And the Precedential Opinion Panel is asked by United Therapeutics. United says, "Hey, Liquidia mentioned this whole research aide argument, backdating, bootstrapping this date that precedes the critical date. Can you guys rule on this?".
And the Precedential Opinion Panel says, "We find that the PTAB, the ALJ's, the administrative law judges, which are not really judges, we can get into that. The 3 judges, that issued their opinion on this at the PTAB, they they didn't show their work. They didn't say why any of these arguments was or was not sufficient. And so, we're not going to deem this a Precedential case. We're not going to make a ruling on this, but we're going to send it back to you guys. You need to clarify this because it was sort of sloppily done.".
Andrew: So, the PTAB's Ruling said the 793 patent is invalid. It is almost as simple as the PTAB going back and saying "The 793 patent is invalid because we agree with Liquidia's primary argument, that these were available in libraries.". And then they could also say "We disagree with Liquidia's arguments. We don't think that the presentation at the conference was enough. And we don't think that the, as we said, the other things referencing this was enough. So, Liquidia wins on point 1, lose on point 2, lose on point 3. But Liquidia wins on point 1 so the thing's invalid. And now the Precedential ruling can say, "Okay, we agree with that. That's enough.". And now, we can get in the future, presenting at conference is not good enough. Is that kind of what you're saying?
Lionel: Yes, Liquidia only needs to win on one of these points. Any one of them would be sufficient to say "Yes, this was this was published.". So, yes, PTAB, the ALJ's go back, they show their work. And once we have that, we can then move forward with the Federal Circuit appeal and, hopefully, for Liquidia, that would mean affirming the ruling which would then become binding on the Federal District Court. We can talk about... Sorry.
Andrew: No, no, that's great. That was exactly the next question. So at this point, hopefully, we've established why we think the 793 patent is invalid, why we thought it was a good argument, why the Patent Board probably thought it was a good argument. We've probably said that. Let's talk timeline, right? So the next things that happened, there's a lot of appeals that are happening here. The lawyers on both sides are probably going to be pretty happy but let's talk the timeline for the reruling at PTAB, the Precedential Appeal Board and then go into the federal courts and how this all plays out.
Lionel: Yes, the timeline is probably the area that I have the least certainty. So, I've gotten a lot of questions around this from a lot of people in
Twitter DM's, and through Substack. And the reason I have uncertainty around this is a few things. One, timelines have really changed in the last couple of years because of COVID. There was a backlog of cases and they had to kind of wade through all of these different cases which had kind of piled up. We've got the holidays now and that is sort of going to delay things a little bit.
Andrew: Good luck getting a federal judge to start a huge new trial between December 25th and January 1st. Good luck.
Lionel: Not a federal judge, at this point. The government employees, basically, ALJ's, right? I mean, these are people who were, maybe, academics or practitioners and then kind of want, a bit of a cushy government job lifestyle. So, they're typically not applying urgency to these things. So, the holidays, actually, it's a pretty significant factor that can delay by a month. There are other sort of convoluting factors I would have expected. I mean, United asked for a rehearing back in August. The Precedential Opinion Panel said to send their piece at the end of October. I would have expected, typically, that we would have had the ALJ's reissue the opinion within a month and it didn't happen. So, I was wrong in my assumed timeline.
Now, once we get that opinion, which at this point, could be mid-January, we're going to have a few months of briefing Federal Circuit appeals. And then there's going to be oral arguments. The decision itself usually happens pretty quickly. After all that happens, I think, from oral argument to decision for non-precedential cases is something like 40 or 42 days. So, that should hopefully happen pretty quickly. I think, if you look at the SEC filings for Liquidia they say, "If everything goes right with PTAB and Federal Circuit, we expect to commercialize mid-2024.
And I think that's a reasonable, comfortable time. I think they've given themselves a little bit of a buffer. And so, I'm increasingly hesitant to apply strict timelines and expectations because of all these convoluted factors. And just say, I think Liquidia, they've been advised by counsel. Cooley is very competent counsel. They know exactly how this stuff plays out at the time. And I think commercialization in 2024 is a pretty comfortable assumption should things go their way with the PTAB and the Federal Circuit.
Andrew: So, I want to talk about the odds of PTAB, but before I do that, I just want to quickly mention, you said mid '24 as commercialization date. And I know some people who agree with you and they think, "Oh, Liquidia's given themselves a little buffer. If things go right and a little bit on the speedy side, it could be end of '23, it could be early '24. But there is one thing that I just want to address and you've been the first person who's called it out. The 793 patent expires in 2027, right? So, if Liquidia comes online mid '24... Liquidia can come online in 2027 when the patent expires without issue, at this point? If Liquidia comes online mid '24 versus early '24 versus late '23, every month matters to UTHR, right?
This is, as I said at the beginning, this is a billion-dollar, run rate drug with incredibly high gross margins. If you get an extra month of exclusivity by dragging this out, one way or another, that's an extra $100,000,000 in revenue? That matters. So, I just want people to keep in their mind, United, not only are they doing everything obviously to try to win but they're going to use every trick they have to try to just delay because a couple extra, even million of lawyers' fees, is worth it if you can get an extra 4 days of exclusivity here. You can add anything there or we can go to the odds if you want.
Lionel: Yes, I think, something else we can talk about if we have time, United has also filed a trade secret misappropriation action in North Carolina. Alleging that a former Liquidia employee, Robert Roscigno, basically took trade secrets from United and used those to help develop the Yutrepia, inhaled Treprosinil program at Liquidia. I've had a lot of questions about that. I think that is another delay tactic for exactly the reasons that you mentioned. In addition to the patent litigation, they are trying to pursue other avenues to prevent the Liquidia from getting on the market, because every day is a couple million bucks.
Andrew: That's great. I know you've done great work, you've given both the base rates for the success at PTAB and everything. So, why don't we just go through the base rates and then we can talk about, if we think Liquidia, based on everything we've talked about, has better or worse odds than the base rates here.
Lionel: Yes, the Federal Circuit, despite the fact that they have patent expertise, they have exclusive jurisdiction over patent appeals. They really don't like to overturn the PTAB. They sort of say, "Hey, that's your domain. If you're telling us that a patent is now invalid and you shouldn't have issued this patents, we're going to believe you.". And so, a lot of people get upset by that. They call this new IPR process of the PTAB, they say these ALJ's are the patent execution, they're the Executioner's, they're out to kill patents. Even despite that, the Federal Circuit really doesn't like to overturn the PTAB. And so, what we see is like 75%-80% of the time, they will more or less affirm the entirety of a PTAB decision.
So, just statistically speaking, I think Liquidia's got a great chance of the 793 invalidation being held up on appeal. I think we can adjust that number upwards for a lot of the reasons that we were just talking about a minute ago. And I think these are good arguments that Liquidia has. We're not having to combine references for an obvious [inaudible]. Typically in obviousness, you say, "Oh, somebody had made a container and somebody else made a wheel and so, the wheelbarrow is obvious. It would be obvious to combine these things and it requires a little bit of an inferential deep, and we don't have that here. We have the inventor himself disclosing his own invention." And so, there is no analysis around whether or not these references actually disclose the Invention. They do. They disclose the invention. It's really just a question of were they available? And again, for the reasons that we just talked about, I think Liquidia's in a really good position.
Andrew: Right. So, the base rate would be 3 and 4, 4 and 5 chance of them winning. And again, as we talked about, if you agree with the librarian who says, "Hey, these were available in very public libraries in 2004, and UTHR's expert is not a librarian so... What you're going to do?. It seems like Liquidia shouldn win this.". Okay, great. I have so many notes and we are running long but I do just want to quickly talk about, I think, at this point, unless you have anything else on the patent, I think we've really covered well. We've got the timeline, we've got all the arguments there, we've got how we think this plays out. It seems both you and I believe that Liquidia has a very, very good chance of winning here. Again, base rate 75%, this seems better than base rate... It seems very good that Liquidia will win and be monetizing by mid '24. Do you agree with everything there? Anything else on the patent?
Lionel: No, I think that's a fair summary.
Andrew: So, I do just want to talk about a couple things. So, let's say neither you and I are healthcare experts, a lot has changed, a lot is always changing in healthcare. A potential competitor drug which I've also just last week stumbled. So, that's one guy but you never know when there's going to be medical breakthroughs, all that. But let's quickly talk about other things at Liquidia. Liquidia wins and mid '23, end of '23, PTAB rules invalid, upheld at federal court, Liquidia comes out and says "Hey, in four months we're going to be on the market.". What do you think the fundamental upside for Liquidia is?
Lionel: Well, I think, we kind of have to go back to the patient pools to analyze this and give the right analysis. So, we have about 12,000 of these patients in the PAH market. It could be 30,000, we have probably, at least at least 12,000. We also have this pulmonary hypertension with interstitial lung disease. We have atleast 30,000 patients. There may be upwards of 60,000. One of the really important things about the Liquidia is that they entered into a promotional agreement with Sandoz for another form of Treprostinil. They are essentially trying to build out a suite of not just the inhaler, but the oral and the injections as well. Now, the revenue coming off of this Sandoz partnership is negligible.
But the important aspect of it is that they're in the market, there talking to payers, they're talking to doctors. They've got a foothold now, so that when they actually do go to commercialize, they will be commercializing really quickly. And I think they're in a great position with potential duopoly, sort of, market to gain upwards of 40% of the market share within a year or 2. And each one of those patients is worth probably a quarter of a million bucks in revenue per year for Liquidia. And so, you can do the math. I mean, this is potentially a couple billion dollar market cap company by 2025.
Andrew: One thing that a shareholder told me when I was talking and prepping for this podcast, they said, "Look, Liquidia...", and I was going to mention this next but might as well mention now, Liquidia's CEO is Roger Jeffs. Roger Jeffs was the CMO at UTHR. He's the one who got UTHR, which is the big-time, [inaudible] competitor. He's the one who did all the work there, got Tyvaso commercialized, all that sort of stuff. So, he saw something with Liquidia's product. Liquidia had a history with CRL's and everything. Once the FDA approved this product, he decided "I want to be the CEO of this company.", that's a signal.
But another thing they said is, "Look, the patient base here, it is very small. There are only about 50 places that are kind of prescribing and treating patients here. And if you look at Liquidia salesforce, because they've already got their salesforce mainly built up. Their salesforce is 50 people and they were saying, "That is not a coincidence. Having one salesperson for every one prescriber, that number is not a coincidence. These guys are ready to go. And the patient base here, a lot of people here orphan drugs. And an orphan drug, it is something that is literally saving your life and you will see...
If it's something where the patient's been on it for 20 years, even if something new and better comes out, they might be hesitant to switch over to the new and better drug, because they've been living on this old drug for 20 years. With Tyvaso, the patients are only on it for about 1.7 years or about 2 years. So, you've kind of got an ideal scenario for orphan drug taking share, where the patient base is turning over reasonably quickly. You have a small number of prescribers so you're going to have a lot of chances to win new market share, I guess, to quote. I'll pause there. Anything you want to add or comment on there, please, feel free.
Lionel: Yes, I mean, look, it's a sad fact that there is that turnover, right? Because it's patients that are recently diagnosed and then, subsequently, they pass away. And so, it's a sad fact that there is turnover at all, but there is. And it does create a market opportunity as you described. I think the other really important point though, is that there is substantial evidence of difference in efficacy. And the patient response is going to be different between Tyvaso and Yutrepia.
And part of that, or should I say a substantial amount of that is due to the manufacturing method that Liquidia is employing. They have a proprietary manufacturing methodology that they call [inaudible], which is essentially a way to create a mold for small particles and make sure that this inhaled powder has really uniform, really small particles. And what that ultimately means for patients is that you're likely to see better penetration into the lungs. They can also redeliver higher dosage and you could have the other benefits like, it reduces cough, it reduces pain in your throat when you inhale this.
And so, not only do you have this turnover and through the market dynamics that you described, but you actually have a product that has differentiated effects. And I think patients and doctors are dying for choice. I talked to employees in Liquidia, I've talked to doctors and there's not a lot out there. So, the mere fact that there is a differentiated product means that Liquidia is in a great position to start gaining market share rapidly.
Andrew: Yes. And just to add on to your point, you've got these finer particles and because of that, you can inhale. And I think Liquidia, they did it in the study, they did it up to like 5 times what Tyvaso's dosage is. But they said, "Hey, there was no limit to this. We could do more.", and that's critical for all sorts of reasons. I'm not a scientist but, basically, what happens with Tyvaso, the current UTHR drug, is eventually your symptoms get worse and you can't inhale enough. So, you have to go on the IV. If you can inhale 10x the dosage on Liquidia, and I just pulled the 10x out of nowhere, well
then you can stay on the Liquidia product for a lot longer.
Inhaling something versus taking it intravenously is going to be a huge quality of life improvement. If you can help the dosage, but with inhaler, maybe you can inhale less than the UTHR product. So, there are a lot of reasons that this product could be superior. And I do think that also comes into a common question, that people are going to have, "Hey, if Liquidia invalidates all of UTHR's patents. Why aren't we going to see just generics flood this market and price in... If you're doing 10,000 patients at 100,000 a year, that's one thing. But if you're doing 10,000 patients and there's 7 products on the market, pricing is going to come down.
So, it's going to be great for patients, going to be great for healthcare. I'd love that for all drugs. But, it's not going to be good for your shareholders. And I think one of the reasons that won't happen is Liquidia actually seems to have a superior product. So, they've got a superior product, patent-protected, unique methods. It's going to kind of, give them advantage of that. I'll let you add anything you want there?
Lionel: Yes, before I talk about the likelihood of other generics, I've got a lot of questions about the data saying that Liquidia is better, and a lot of it is from Yutrepia, that's Liquidia's product, Yutrepia to United's nebulizer [inaudible]. And so, you have to extrapolate a little bit to kind of do a DPI to DPI comparison. And so, that is a valid criticism of the analysis or things that check, you need a gut check on this, say "Okay, is the DPI, is the dry powder inhaler really going to be better than Tyvaso?". And you're most likely not going to see a DPI to DPI study for a few reasons.
One, I think , Liquidia is in a great position to gain market share for the reasons that we already talked about. And so, I don't think it's super necessary. Two, Tyvaso is heavily controlled. In order to do this study, Liquidia would actually have to get the product. And getting the product is not easy, but you have to be patient. And so it's sort of a valid criticism or valid gut-check to say, "Hey, this isn't apples-to-apples comparison. Can you actually say, you trapeze better?". But again, for the reasons that we just talked about, this print technology and a lot of the circumstantial evidence around patient tolerance, there's a lot of indication a lot of reason to believe that it is, in fact, better.
Now, to your next point about generics. Are generics going to flood the market? There are a number of different avenues for for treating pulmonary hypertension depending on what group you're in. There's 5 groups, they call them WHO groups, 1 through 5. Not all of the methods out there are suited for pulmonary arterial hypertension and pulmonary hypertension with interstitial lung disease, those are the indications that this inhaled Treprostinil is being used to treat. Even if other generic manufacturers wanted to flood the market, Liquidea has not just a patent protection on their specific print technology or whatever they're manufacturing methods. They have a lot of trade secret knowledge around how to manufacture their dry powder Treprostinil.
And one of the biggest issues with the dry powder is that it's super hygroscopic, that means it pulls water out of the air and essentially becomes unusable, because you don't want to be inhaling water in your lungs, right? And that is something that Liquidia has been working on since like 2015. They spent at least 5 years trying to really address this one issue of how do you create a stable Treprostinil compound that is not just stable but stable at room temperature so that it's easy to use, transport, to store, et cetera.
That is not a trivial problem to solve and so, because of this patent. overhang, what I highly suspect is the case, is that all these other manufacturers are thinking, "Why am I going to invest a bunch of money in R&D, if these United patents might be valid until 2027? We don't have clarity, we don't know if anyone can get into the market. Even if it's valuable, it's just not worth our time.". And so, if Liquidia gets the win at the PTAB and the Federal Circuit, they're in a really good position to have a massive head start. And again, I've talked to employees, I've talked to others in the industry who's saying this is a really significant, this hygroscopicity issue is a really significant issue. And so, I don't think you're going to see a flood of generics because the R&D lift is huge.
Andrew: I completely agree. We are running long and my dog is letting me know it's time for her to go to the restroom. So, we're probably gonna have to wrap it up here, but I just want to do 2 quick things. Agree with me, disagree with me and then I want to turn it over to you for final thoughts, get it out, if any and then we'll wrap it up. 2 quick things. Number one, it is very hard to assign like, "Oh, this is a cable company. They're going to do $5 in cash flow next year, put a 10x multiple $50.". It's very hard but I do think a very simple way would be to say Tyvaso will probably be doing about 1.2 billion in sales by the time Liquidia comes online.
Every 10% of the market that you think Liquidia could do, would be $120 if you're going to slap a $120 in revenue. If you're going to slap a 4 times multiple on that, that means every 10% of the market they get is worth about $4 per share if you slap [inaudible] revenue multiple, you get the picture. Now, obviously you've got to think, when the generics come, when does pricing... All of that sort of stuff. But I think that's a very simple way. I know a lot of people who look at it and say "They're going to get way more than 50% in the market.". But if you think they get about 50%, then you're talking "Hey, this is $20 stock plus whatever cash they have on the balance sheet.". You can agree, disagree. I know people who think the technology is going to be worth a lot more in the long run, all that sort of stuff, but anything you want to add on there?
Lionel: No, I think that's that's a pretty reasonable kind of base case. Look, Liquidia is not talking about pricing. That information is not publicly available and so, it's really going to come down to, do they have to undercut, Tyvaso, in any capacity? Or are the added benefits that we talked about, that are derivative of the print technology, do those provide enough benefit that they can actually be on parity with the Tyvaso price?
Andrew: Or maybe premium. But I think premium might be hard for orphan drugs. And then the last thing, a lot of people are worried about cash runway. And so, they commercialize, this going to be a pretty heavily cash burning
company to me and feel free to tell me if you disagree. They did a raise in April. They've got about 100 million in cash on the balance sheet. They will burn through that reasonably quickly with the legal fees and all the startup fees but they've got enough to cover at least '23-'24.
I think their kind of line is, "We've got enough to cover us through getting to commercialization.". Look, it's never fun to say they're going to have to raise at some point. But I think they get to commercialization or they get line-of-sight to commercialization, the stock is, hopefully, probably a lot higher when that happens. And then you can kind of address it through a stock raise, partnering with another company. I think the critical point here on Liquidia is they've got enough to see this trial through and have line-of-sight to commercialization.
Lionel: Yes, I think that's right. And I talked to guys on Liquidia team and I know that they're looking at also non-dilutive ways to go about funding. So, they haven't committed to anything but they are exploring all of their options. And like you say, even if they do have to raise, if they have that line-of-sight, I think it bodes well for the stock.
Andrew: Okay. Well, Lionel, this has been absolutely fantastic. Again, this is one of the most interesting ones I've come across in a while. I know you and I, we've been talking about it for months. I'm glad we could get this podcast out there. I think it's a real banger. I remind everyone, this is lots and lots of risk here, but hopefully, I hope we've done good analysis here. But everybody should think through all those risks very carefully. Remember, this is an investing advice. But Lionel, appreciate you wearing the hat. I hope you have a great holidays and looking forward to having you on again in the new year.
Lionel: Thank you so much. Always loved being on the podcast. Appreciate you having me on for a second time and I hope you have a great holiday season as well.
Andrew: We'll talk to you, buddy.
Lionel: Bye.
[END]